

WE INSPIRE!
With VSY Biotechnology, you can rely on over 15 years of experience in science driven development, manufacturing, and marketing of innovative medical products.
Our orthopedic portfolio covers a wide and unique range of biotechnologically produced intra-articular hyaluronic acid injection products for the treatment of osteoarthritis; all of them produced under the highest industry standards, certified across the globe and clinically proven to be safe, effective and well tolerated by patients around the globe!
10 years of Reviscon® - Motion is life!
Since the market launch of Reviscon® in 2015, we have continuously expanded our innovative orthopedic portfolio and set new standards in the treatment of osteoarthritis with our unique technology. Our research approach is focused exclusively on the well-being of the patient. VSY Biotechnology´s osteoarthritis therapy with Reviscon® is nowadays used worldwide and helps patients around the globe to improve their quality of life by reducing pain and improving overall mobility
The Reviscon® promise - Focus on the patient!
Our continuous investment in research and development of disruptive therapies for the treatment of musculoskeletal diseases reflects VSY Biotechnology's approach of using meticulous scientific precision to tackle the spread of one of the most challenging health problems of our time - osteoarthritis of the joints! A clinically proven, symptom-oriented therapy is intra-articular injection with our hyaluronic acid preparation Reviscon®. This treatment method has been an integral part of global physician´s every day’s clinical practice for many years, with a clear focus on the patient.
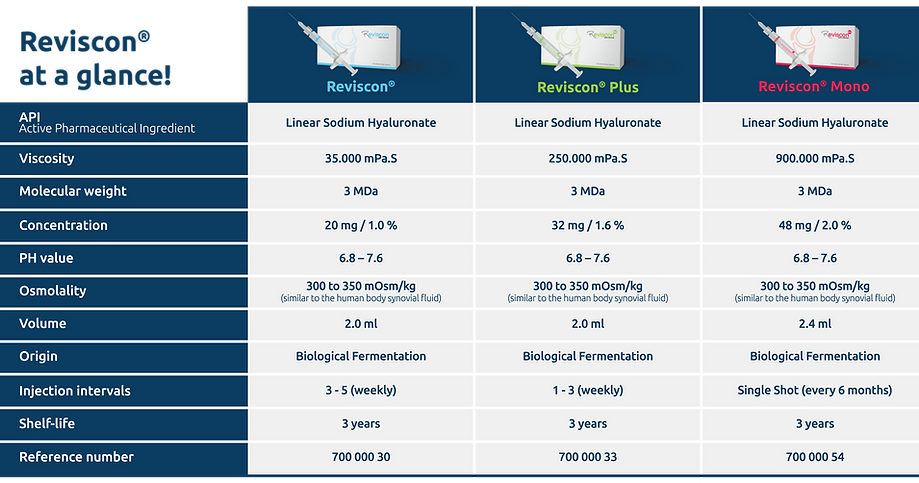
Reviscon®

Sophisticated product design resulting in therapeutic excellence!

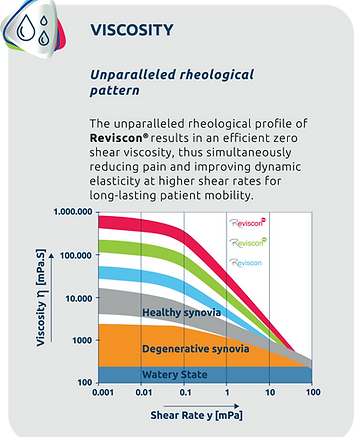
The Reviscon® formula
Sophisticated product design resulting in therapeutic excellence!
Reviscon® contains a structured, linear sodium hyaluronate (NaHa) pattern. This unique formulation and the scientifically designed, highly sophisticated interplay between viscosity, molecular weight and hyaluronic acid concentration complement each other in their mode of action and play a pivotal role in joint care solutions, providing patients with a potent and efficient remedy for joint-related concerns.


Reviscon® clinical evidence
A recent study[1] carried out with Reviscon®, Reviscon® Plus and Reviscon® Mono proved that intra-articular injections for osteoarthritis with the Reviscon® sodium hyaluronate portfolio and different doses of linear high-molecular weight hyaluronic acid can improve pain, stiffness, function, and quality of life in patients suffering from OA over a six-month period.
---Reviscon® - clinically proven and effective up to 6 months!---



features & benefits
The trailblazer in cross-linked single-shot OA therapy!

With a molecular weight of 1 Million Dalton (MDa) Re-Cross®, coupled with the meticulously designed HXL HYBRID XLink TechnologyTM, Re-Cross® IA-HA is tailored for rest and physical activity, and aligns with the dynamic nature of joint movement. The cross-linked NaHa degrades slower than the linear NaHa, thereby providing unique cohesive energy characteristics, increased bioavailability, and thus prolonged pain relief. Additionally, the combination of linear and cross-linked NaHa helps minimizing further cartilage damage and fosters a healthy joint environment by its anti-inflammatory effect.
HXL HYBRID XLink TechnologyTM


Re-Cross® 2.2% (88 mg) is a non-pyrogenic, non-immunogenic, highly purified, cross-linked sodium hyaluronate (NaHa) solution for intra-articular injections into the synovial joints that is meticulously
crafted for precision and purity, catering to individuals seeking a targeted and refined approach to joint health.
Re-Cross® IA-HA is biotechnologically manufactured (microbiological fermentation) according to ISO 13485 and does not contain any preservatives or ingredients from biological sources.
Highly purified NaHa solution

Re-Cross® 2.2% (88 mg) sodium hyaluronate (NaHa) is powered by VSY Biotechnology´s proprietary HXL HYBRID XLink TechnologyTM. By administrating a combination of the dispersive, linear, and cohesive, cross-linked NaHa molecule chains into the synovial joints, both, mechanical strength, and shock absorbing properties as well lubricating and joint cushioning efficacy are enhanced, especially in case of articular cartilage damage.
Linear and cross-linked molecule structures


Re-Cross® 2.2% (88 mg) sodium hyaluronate is a hyaluronic acid with 1 MDa, effectively
enhancing shock absorption in the synovial joints. The mixed patterns of linear and cross-linked molecule chains in Re-Cross® provides both, a high lubricating layer on the surrounding cartilage and tissue as well as an increased shock absorption effect, supporting the weight bearing capacities of the affected synovial joints. Simultaneously, Re-Cross® diminishes friction, enhances joint mobility, and provides long term pain relief between 6 to 12 months. [1]
Enhanced lubricating- and shock absorbing properties
Re-Cross® 2.2% (88 mg) is designed to imitate healthy, natural synovial fluid properties to the
highest extent possible. The biological mimicry of Re-Cross® IA-HA ensures optimal
bioavailability and integration into the joint environment, with minimal degradation, aligning
closely with the natural properties of human synovial fluid. The technology behind Re-Cross®
IA-HA is laid out for patients with early onset OA, and especially for individuals an active
lifestyle, active and leisure athletes with first signs of movement limitations and joint pain.
Mimicking healthy human synovial fluid properties

The Re-Cross® administration protocol of only one syringe every six to twelve months, offers a sustainable and convenient therapy solution for individuals seeking long-lasting joint support and underscores the product’s efficacy in promoting joint health over an extended period. This approach reflects VSY Biotechnology´s commitment to patient centric, well-structured, and effective treatment plans and optimizes the impact of IA-HA OA therapy, allowing for gradual
and sustained improvement in joint health.
Patient centric administration protocol


Intra-articular viscosupplementation into the synovial joints with Re-Cross® sodium
hyaluronate is a safe and efficient procedure for patients suffering from OA and who have not responded sufficiently to previous pharmacologic treatments. To date, zero product related side-effects have been reported[1]. 4
Patient safety and therapy efficacy






